Background: Systemic AL amyloidosis is characterized by the deposition of insoluble amyloid fibrils produced by light chains synthesized by clonal CD38+ plasma cells. Combining daratumumab (DARA) with bortezomib, cyclophosphamide, and dexamethasone (VCd) has demonstrated significantly improved outcomes in patients with AL amyloidosis. The classification of hematologic complete response (CR) in this disease is evolving, and the absolute reduction of the involved free light chain (iFLC) and the difference between iFLC and uninvolved free light chain (dFLC) are being recognized as very meaningful endpoints. Here, we present results from the ANDROMEDA study (NCT03201965) to demonstrate the impact of achieving deep reductions of iFLC and dFLC on major organ deterioration progression-free survival (MOD-PFS), a novel, key secondary endpoint in this study.
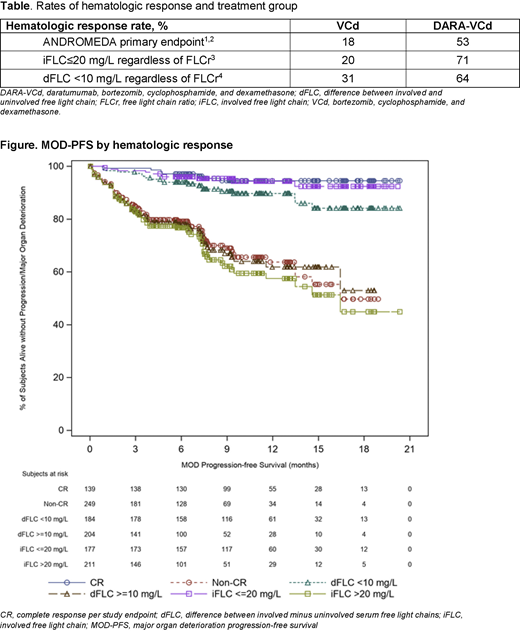
Methods: Key eligibility criteria included newly diagnosed AL amyloidosis with measurable hematologic disease (serum monoclonal protein ≥0.5 g/dL by protein electrophoresis or serum free light chain ≥5.0 mg/dL with an abnormal kappa:lambda ratio or dFLC ≥50 mg/L), ≥1 involved organ, cardiac stage I-IIIA, eGFR ≥20 mL/min, and absence of symptomatic multiple myeloma. Patients were randomized (1:1) to receive DARA-VCd or VCd alone. All patients received bortezomib (1.3 mg/m2 subcutaneous [SC] weekly), cyclophosphamide (300 mg/m2 oral [PO] or intravenous [IV] weekly), and dexamethasone (20-40 mg PO or IV weekly) for six 28-day cycles. DARA SC (1800 mg, co-formulated with recombinant human hyaluronidase PH20 in 15 mL) was administered by injection weekly in Cycles 1-2, every other week in Cycles 3-6, and every 4 weeks thereafter for up to 24 cycles. Disease evaluations occurred every 4 weeks (Cycles 1-6) and every 8 weeks (after Cycle 7) until major organ deterioration, death, end of study, or withdrawal. The primary endpoint was overall (ie, at any time) hematologic CR rate, defined here as normalization of FLC levels and ratio (FLCr) and negative serum and urine immunofixation, confirmed at a subsequent visit; normalization of uninvolved FLC level and FLCr were not required if iFLC<upper limit of normal.1-2 The following criteria for deep hematological response were evaluated: iFLC ≤20 mg/L regardless of FLCr3 and dFLC<10 regardless of FLCr.4 MOD-PFS is a composite endpoint defined as any one of the following events (whichever comes first): death; cardiac deterioration (requiring cardiac transplant, left ventricular assist device or intra-aortic balloon pump); end stage renal disease requiring hemodialysis or renal transplant; or hematologic progression per consensus guidelines.1 Analyses of deep hematological responses were performed on the intent-to-treat analysis set; patients without a baseline assessment or post-baseline assessment were considered non-responders. Descriptive statistics were used to summarize overall response rates. Kaplan-Meier curves were plotted for MOD-PFS by hematologic response status.
Results: A total of 388 patients were randomized to receive DARA-VCd (n=195) or VCd alone (n=193). Baseline characteristics were well balanced between treatment groups. The median age was 64 years and 65% of patients had ≥2 organs involved. The proportions of patients with heart and kidney involvement were 71% and 59%, respectively, and the proportions of patients with cardiac stage I, II, and IIIA were 23%, 40%, and 37%, respectively. The median duration of treatment was 9.6 months for DARA-VCd and 5.3 months for VCd. Median follow-up was 11.4 months (range, 0.03-21.3+). The rates of deep hematological responses by all criteria strongly favored the DARA-VCd treatment arm (Table). MOD-PFS was longer in patients achieving deep hematological responses by all criteria (Figure). In addition, the corresponding MOD-PFS was similar regardless of the hematological response criteria used.
Conclusions: Regardless of the criteria used, the addition of DARA to VCd increased the rates of deep hematologic responses in patients with newly diagnosed AL amyloidosis, which, in turn, was associated with prolonged MOD-PFS. These results support the benefit of DARA in this patient population.
References
1. Comenzo RL, et al. Leukemia. 2012;26(11):2317-25
2. Sidana S, et al. Leukemia. 2019;34(5):1472-75
3. Muchtar E, et al. Leukemia. 2019;33(3):790-94
4. Manwani R, et al. Blood. 2019;134(25):2271-2280
Comenzo:Amgen: Consultancy; Sanofi: Consultancy; Prothena: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; Unum: Consultancy; Karyopharm: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Caleum: Consultancy. Kastritis:Pfizer: Consultancy, Honoraria; Amgen: Consultancy, Honoraria, Research Funding; Genesis Pharma: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria. Palladini:Celgene: Other: Travel support; Jannsen Cilag: Honoraria, Other. Minnema:Celgene: Other: travel support, Research Funding; Kite, a Gilead Company: Speakers Bureau; Amgen: Consultancy; Servier: Consultancy. Wechalekar:Caelum: Other: Advisory; Janssen: Honoraria, Other: Advisory; Takeda: Honoraria, Other: Travel; Celgene: Honoraria. Jaccard:Celgene: Honoraria, Other: A.J. has served in a consulting or advisory role for Janssen and has received honoraria from, received research funding from, and had travel, accommodations, or other expenses paid for or reimbursed by Celgene., Research Funding; Janssen: Consultancy, Honoraria, Other: A.J. has served in a consulting or advisory role for Janssen and has received honoraria from, received research funding from, and had travel, accommodations, or other expenses paid for or reimbursed by Janssen., Research Funding. Sanchorawala:Proclara: Other: advisory board; Abbvie: Other: advisory board; UpToDate: Patents & Royalties; Regeneron: Other: advisory board; Caleum: Other: advisory board; Janssen: Research Funding; Takeda: Research Funding; Celgene: Research Funding; Prothena: Research Funding; Caelum: Research Funding; Oncopeptide: Research Funding. Lee:Janssen: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Genentech: Consultancy; GlaxoSmithKline: Consultancy, Research Funding; Sanofi: Consultancy; Daiichi Sankyo: Research Funding; Regeneron: Research Funding; Genentech: Consultancy; Celgene: Consultancy, Research Funding; Amgen: Consultancy, Research Funding. Gibbs:Janssen, BMS/Celgene, Amgen, Takeda, Pfizer, Caelum, Abbvie and Eidos: Membership on an entity's Board of Directors or advisory committees. Mollee:Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS/Celgene: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Caelum: Membership on an entity's Board of Directors or advisory committees. Venner:Celgene, Amgen: Research Funding; Janssen, BMS/Celgene, Sanofi, Takeda, Amgen: Honoraria. Schönland:Janssen, Prothena, Takeda: Honoraria, Other: travel support to meetings, Research Funding. Suzuki:Takeda, Amgen, Janssen and Celgene: Consultancy; Bristol-Myers Squibb, Celgene and Amgen: Research Funding; Takeda, Celgene, ONO, Amgen, Novartis, Sanofi, Bristol-Myers Squibb, AbbVie and Janssen: Honoraria. Kim:Amgen, BMS, Janssen, Sanofi, Takeda: Consultancy, Honoraria, Research Funding. Cibeira:Janssen, Akcea Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen, Celgene, Amgen: Honoraria, Other: Educational lectures. Beksac:Deva: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sanofi: Speakers Bureau; Amgen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen&janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Valent:Amgen Inc.: Other: Teaching, Speakers Bureau; Takeda Pharmaceuticals: Other: Teaching, Speakers Bureau; Celgene: Other: Teaching, Speakers Bureau. Wong:Fortis: Research Funding; GSK: Research Funding; Amgen: Consultancy; Janssen: Research Funding; Roche: Research Funding; Bristol Myers Squibb: Research Funding; Sanofi: Membership on an entity's Board of Directors or advisory committees. Rosenzweig:Janssen: Speakers Bureau. Bumma:Sanofi: Speakers Bureau; Amgen: Speakers Bureau. Dimopoulos:BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Personal fees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Personal fees, Research Funding, Speakers Bureau; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Personal fees, Speakers Bureau; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Personal fees, Research Funding, Speakers Bureau; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Personal fees, Research Funding, Speakers Bureau. Tran:Janssen: Current Employment, Current equity holder in publicly-traded company. Qin:Janssen: Current Employment. Vasey:Janssen Research & Development: Current Employment, Current equity holder in publicly-traded company. Tromp:Janssen: Current Employment, Current equity holder in publicly-traded company. Weiss:Janssen: Current Employment, Current equity holder in publicly-traded company. Vermeulen:Janssen: Current Employment, Current equity holder in publicly-traded company.
Author notes
Asterisk with author names denotes non-ASH members.